-
2016 International Conference on TPP/RCEP, Medical Products and Food Safety
-
已結束
活動資訊
活動日期:2016-11-10至2016-11-11
- 活動地點:張榮發國際會議中心8樓801會議廳 (台北市中正區中山南路11號)
活動時間:09:10-17:00
- 聯絡方式:李小姐 (02)2321-2362 分機 24
隨著近年來區域經濟整合的盛行,雙邊與多邊自由貿易協定數量不僅快速增加,規模也不斷擴大,與我國息息相關的跨太平洋夥伴協定 (Trans-Pacific Partnership Agreement, TPP)與區域全面經濟夥伴關係協定(Regional Comprehensive Economic Partnership, RCEP)也成為近年國內各領域探討的重點議題。
TPP與RCEP成員國涵蓋泛太平洋及亞洲地區數個重要國家,TPP已於2016年2月正式簽訂,而RCEP截至2016年8月為止已完成第14回合談判,為建構我國與國際醫藥法規接軌,並拓展國際醫藥衛生合作機會,衛生福利部食品藥物管理署規劃此「2016年區域自由貿易協定與醫藥衛生政策國際研討會系列」(2016 International Conference on TPP/RCEP, Medical Products and Food Safety),邀請美國、日本、澳洲及東南亞等各國及國內政府、學界及產業界專家針對TPP與RCEP等區域自由貿易協定關於醫藥、食品安全議題進行深度探討,以掌握最新國際情勢並促進本國與各國的資訊交流。
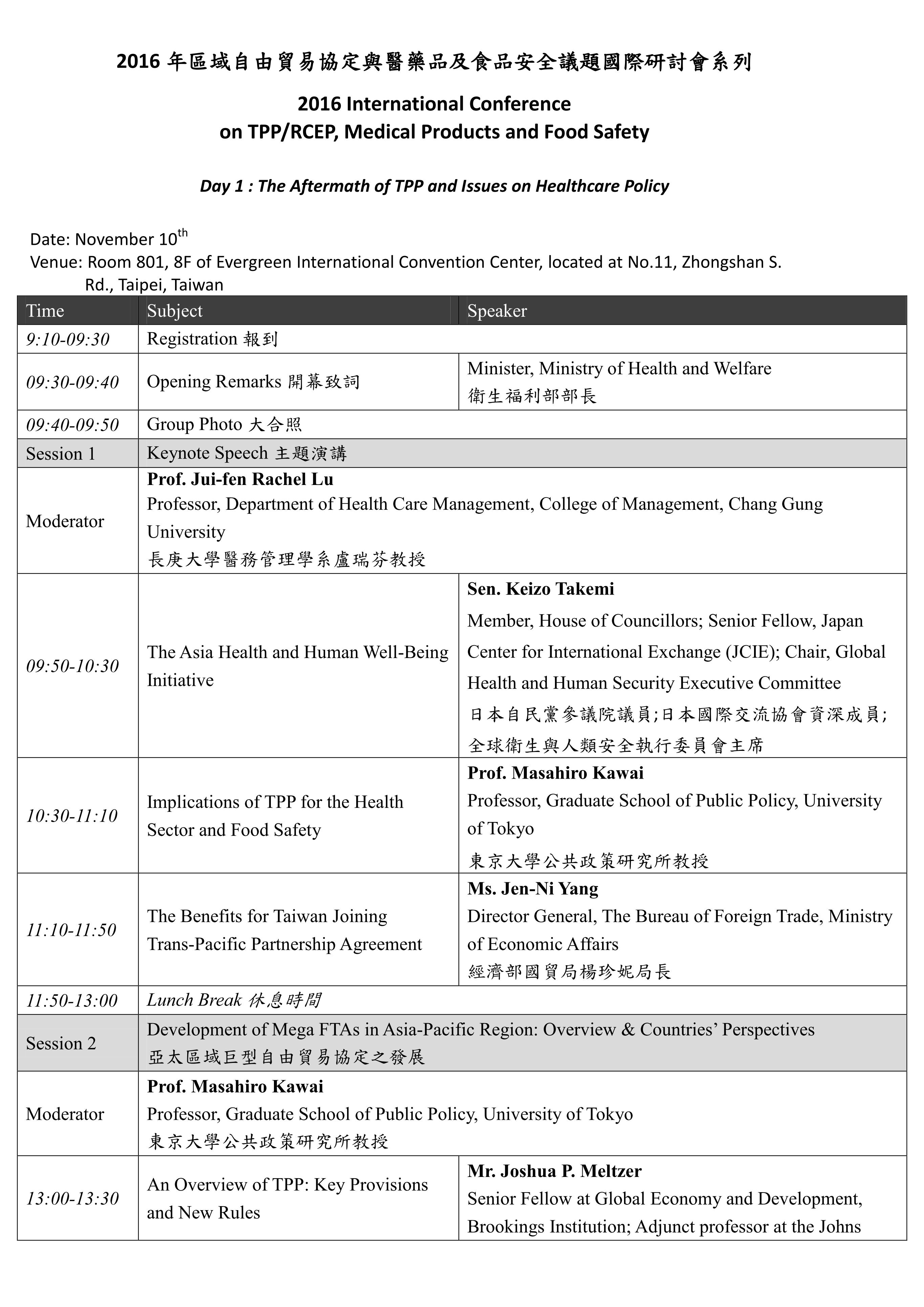
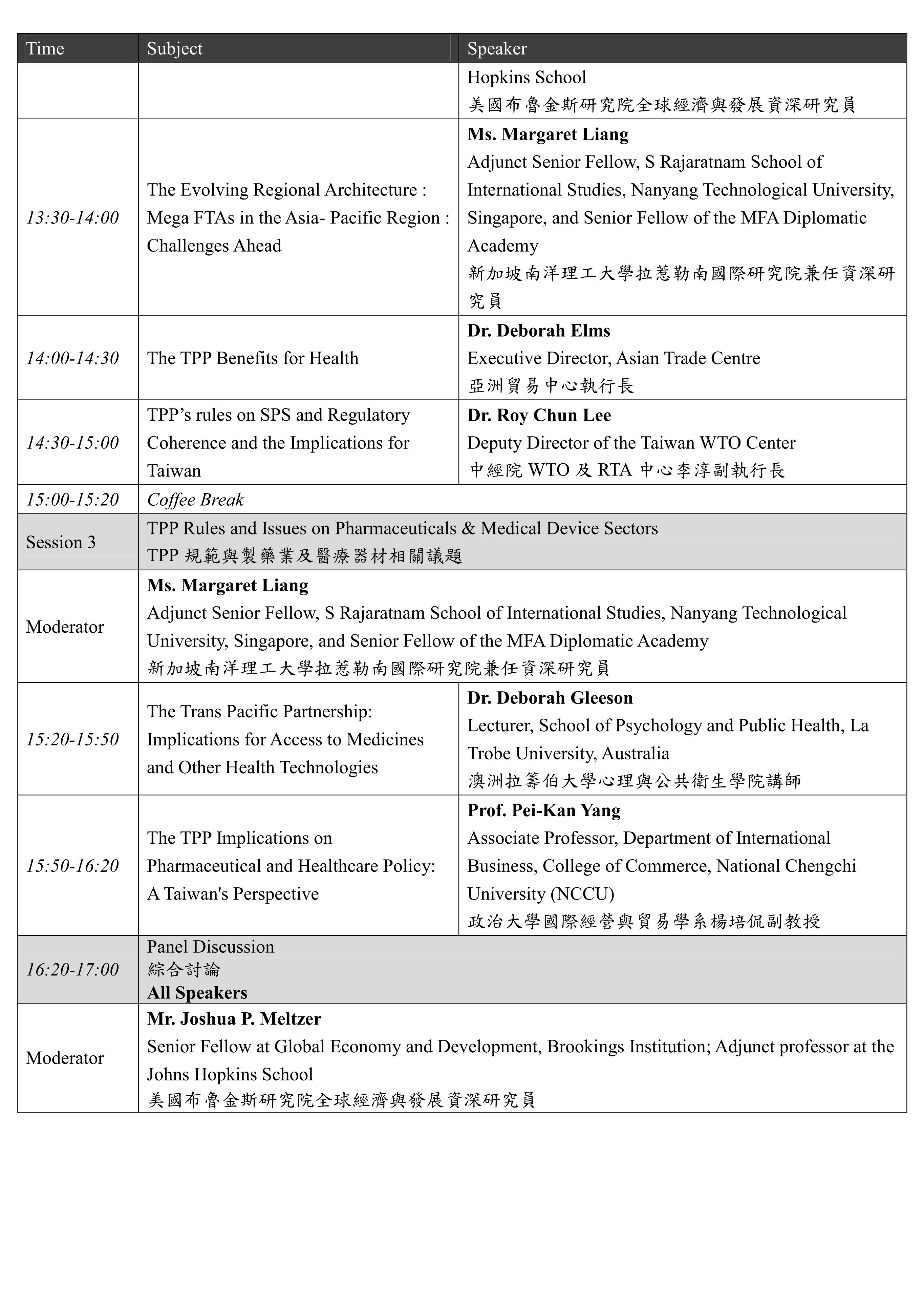
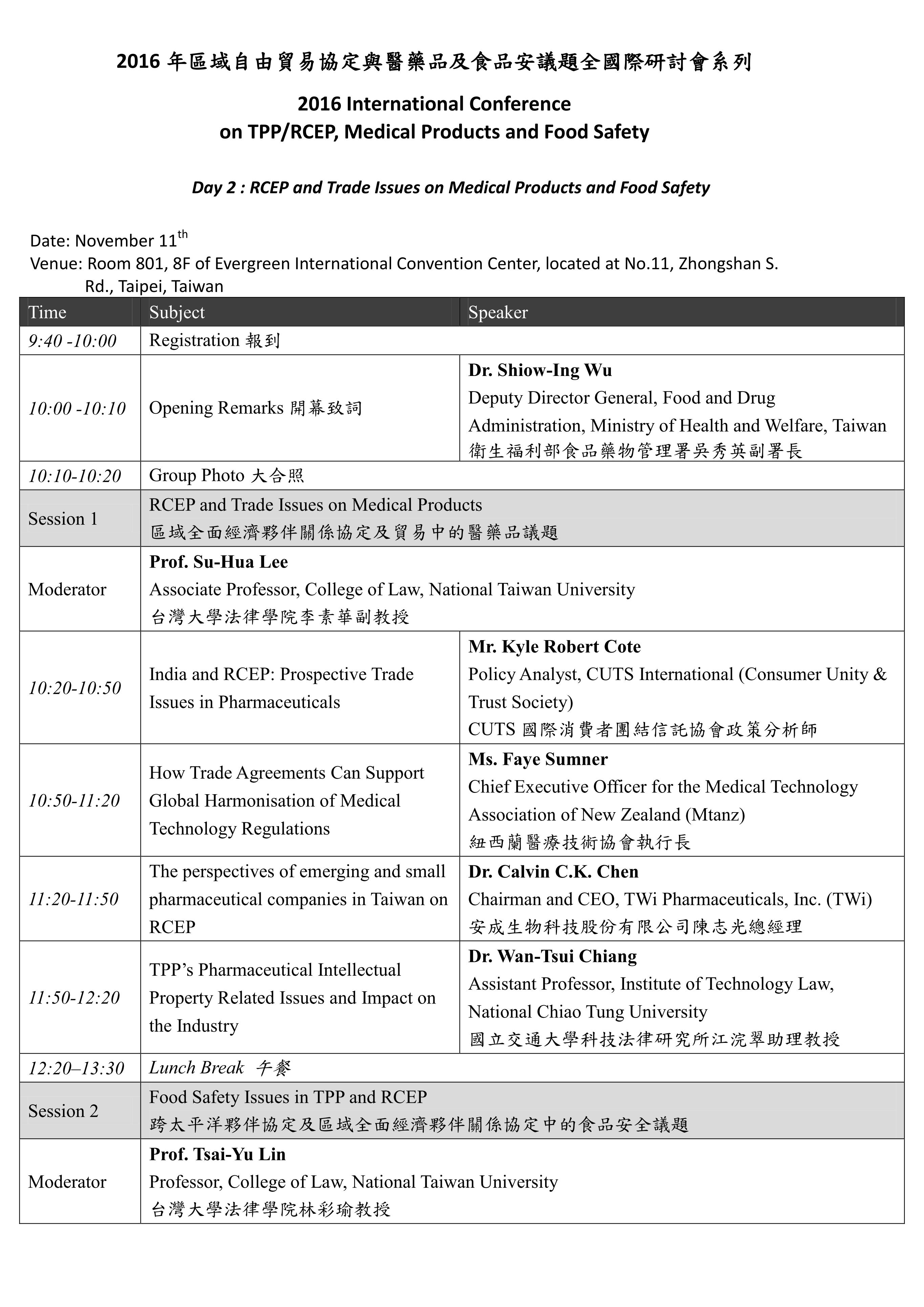
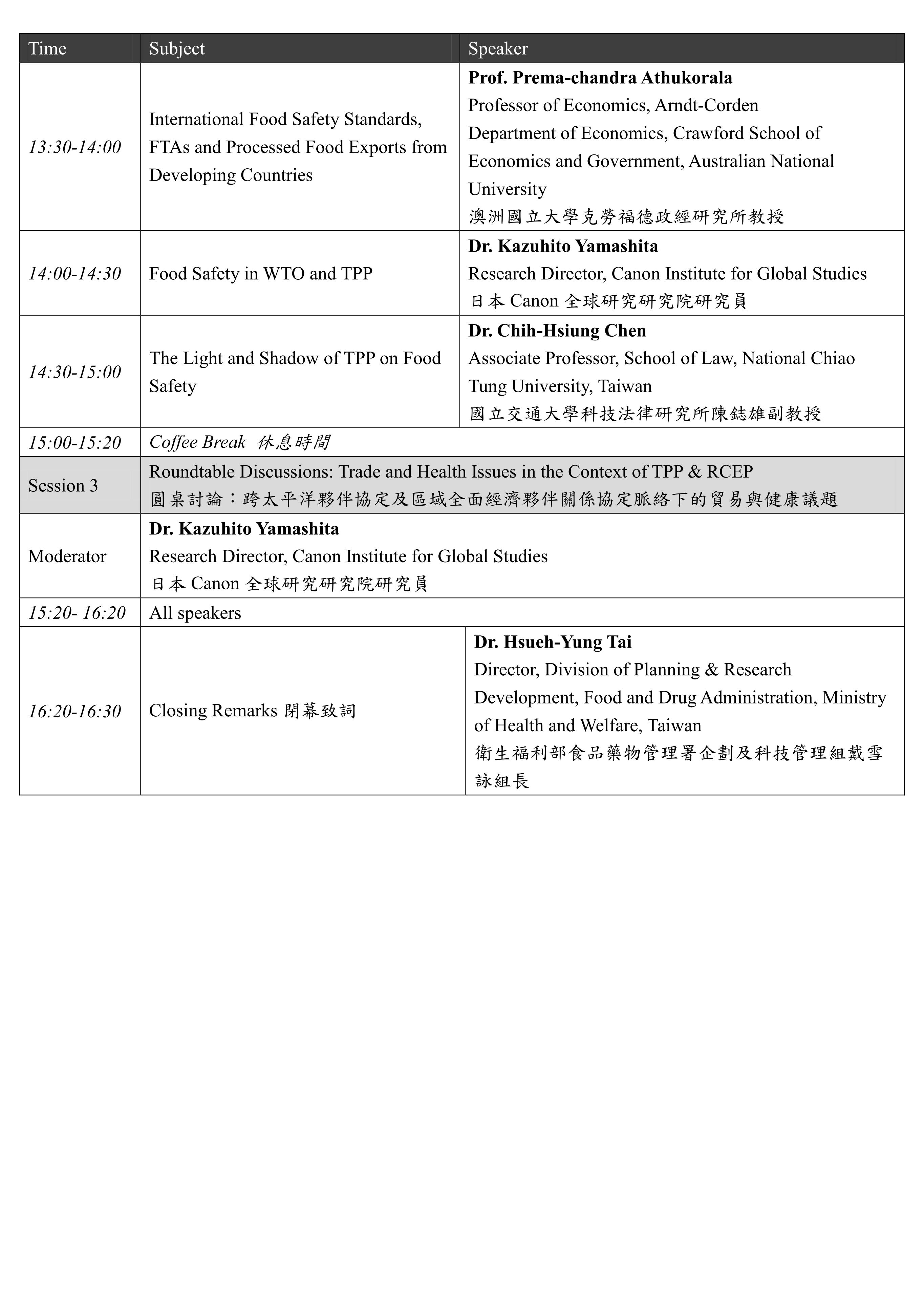
本系列國際研討會預計於11月10-11日辦理,共分為兩個場次,主題分別為「The Aftermath of TPP and Issues on Healthcare Policy」及「RCEP and Trade Issues on Medical Products and Food Safety」。「The Aftermath of TPP and Issues on Healthcare Policy」將針對「Development of Mega FTAs in Asia-Pacific Region: Overview & Countries’ Perspectives」、「TPP Rules and Issues on Pharmaceuticals & Medical Device Sectors」等主題進行討論;「RCEP and Trade Issues on Medical Products and Food Safety」將針對「RCEP and Trade Issues on Medical Policy」及「Food Safety Issues in TPP and RCEP」等主題進行交流。期望透過本次研討會之辦理,了解當前區域貿易協定中有關醫藥衛生議題之發展趨勢,亦期望參考區域內相關國家之經驗,以集思廣益我國未來相關政策與法規建置之方向。
As the regional economic integration prevails recently, bilateral and multilateral trade agreements are not only increased rapidly but also enlarge the scales. As a result, both the Trans-Pacific Partnership Agreement (TPP) and the Regional Comprehensive Economic Partnership (RCEP) become the popular issues among individual fields in Taiwan.
The memberships of TPP and RCEP cover the significant states within the Pacific and Asian areas. The TPP agreement was signed in February 2016 and the RCEP has finished its 14th round of negotiations until August 2016. For the purpose of bridging the domestic and international medical regulations as well as exploring the cooperation opportunity internationally, the Food and Drug Administration, Ministry of Health and Welfare, arranges 2016 International Conference on TPP/RCEP, Medical Products and Food Safety, which invites experts from the United States, Japan, Australia, Southeast Asia, and Taiwan to discuss the latest information regarding medical and food safety issues within the TPP and RCEP as well as promote the information exchange among related states.
The Conference will be held on 10-11th November with two subjects entitled “The Aftermath of TPP and Issues on Healthcare Policy” and “RCEP and Trade Issues on Medical Products and Food Safety.” Through this conference, it is expected to understand the current regional trade agreements as well as its development trend related to domestic medical policy; and it is also expected to learn the relevant experiences and suggestions from related states in order to assist in directing the domestic policies and regulations in the future.
本活動採網路免費報名,因場地限制,人數有限,額滿為止。
本次會議提供口譯服務。
請使用Google Chrome 瀏覽器或最新版IE瀏覽器(IE10以上)報名。
活動地點
張榮發國際會議中心8樓801會議廳 (台北市中正區中山南路11號)
隨著近年來區域經濟整合的盛行,雙邊與多邊自由貿易協定數量不僅快速增加,規模也不斷擴大,與我國息息相關的跨太平洋夥伴協定 (Trans-Pacific Partnership Agreement, TPP)與區域全面經濟夥伴關係協定(Regional Comprehensive Economic Partnership, RCEP)也成為近年國內各領域探討的重點議題。
TPP與RCEP成員國涵蓋泛太平洋及亞洲地區數個重要國家,TPP已於2016年2月正式簽訂,而RCEP截至2016年8月為止已完成第14回合談判,為建構我國與國際醫藥法規接軌,並拓展國際醫藥衛生合作機會,衛生福利部食品藥物管理署規劃此「2016年區域自由貿易協定與醫藥衛生政策國際研討會系列」(2016 International Conference on TPP/RCEP, Medical Products and Food Safety),邀請美國、日本、澳洲及東南亞等各國及國內政府、學界及產業界專家針對TPP與RCEP等區域自由貿易協定關於醫藥、食品安全議題進行深度探討,以掌握最新國際情勢並促進本國與各國的資訊交流。
本系列國際研討會預計於11月10-11日辦理,共分為兩個場次,主題分別為「The Aftermath of TPP and Issues on Healthcare Policy」及「RCEP and Trade Issues on Medical Products and Food Safety」。「The Aftermath of TPP and Issues on Healthcare Policy」將針對「Development of Mega FTAs in Asia-Pacific Region: Overview & Countries’ Perspectives」、「TPP Rules and Issues on Pharmaceuticals & Medical Device Sectors」等主題進行討論;「RCEP and Trade Issues on Medical Products and Food Safety」將針對「RCEP and Trade Issues on Medical Policy」及「Food Safety Issues in TPP and RCEP」等主題進行交流。期望透過本次研討會之辦理,了解當前區域貿易協定中有關醫藥衛生議題之發展趨勢,亦期望參考區域內相關國家之經驗,以集思廣益我國未來相關政策與法規建置之方向。
As the regional economic integration prevails recently, bilateral and multilateral trade agreements are not only increased rapidly but also enlarge the scales. As a result, both the Trans-Pacific Partnership Agreement (TPP) and the Regional Comprehensive Economic Partnership (RCEP) become the popular issues among individual fields in Taiwan.
The memberships of TPP and RCEP cover the significant states within the Pacific and Asian areas. The TPP agreement was signed in February 2016 and the RCEP has finished its 14th round of negotiations until August 2016. For the purpose of bridging the domestic and international medical regulations as well as exploring the cooperation opportunity internationally, the Food and Drug Administration, Ministry of Health and Welfare, arranges 2016 International Conference on TPP/RCEP, Medical Products and Food Safety, which invites experts from the United States, Japan, Australia, Southeast Asia, and Taiwan to discuss the latest information regarding medical and food safety issues within the TPP and RCEP as well as promote the information exchange among related states.
The Conference will be held on 10-11th November with two subjects entitled “The Aftermath of TPP and Issues on Healthcare Policy” and “RCEP and Trade Issues on Medical Products and Food Safety.” Through this conference, it is expected to understand the current regional trade agreements as well as its development trend related to domestic medical policy; and it is also expected to learn the relevant experiences and suggestions from related states in order to assist in directing the domestic policies and regulations in the future.
本活動採網路免費報名,因場地限制,人數有限,額滿為止。
本次會議提供口譯服務。
請使用Google Chrome 瀏覽器或最新版IE瀏覽器(IE10以上)報名。




活動地點
張榮發國際會議中心8樓801會議廳 (台北市中正區中山南路11號)



